3. Chemical Process Industries
| Site: | MSubbu Academy |
| Course: | Introduction to Chemical Engineering |
| Book: | 3. Chemical Process Industries |
| Printed by: | Guest user |
| Date: | Friday, 4 April 2025, 2:11 PM |
Description
Objectives
To given an idea of process flow sheet, and about chemical process based on industrial manufacture of sulfuric acid and soda ash.Process Flow Diagram
A process flow diagram (PFD) is a diagram commonly used in chemical engineering to indicate the general flow of chemicals and connection of equipments. The PFD contains the bulk of the chemical engineering data necessary for the design of a chemical process. The PFD displays the relationship between major equipment of a plant facility and does not show minor details such as piping details and designations.Sulfuric Acid
Sulfuric acid (\(\ce{H2SO4}\)) is the largest volume industrial chemical produced in the World. The consumption of sulfuric acid is often used to monitor a country’s degree of industrialisation.
It is a viscous, high density liquid (1.83 g/cc), known in antiquity as oil of vitriol.
It is sold as 98% (concentrated), 10% (dilute), or 29–32% (for use in car batteries).
Almost all sulfuric acid in the world today is made by the Contact Process, and so called, because in a key step of the production process, the reactants are in contact with the catalyst — vanadium pentoxide (\(\ce{V2O5}\)).
Industrial Production
In 2018, the global production of sulfuric acid was 270 million ton (in 1997, it was 156 million ton), with India’s share of 6%. Currently, there are more than 65 sulfuric acid plants spread across India. The major raw materials used in the manufacture of sulfuric acid include elemental sulfur, hydrogen sulfide, pyrites, etc. Nearly all factories in India rely heavily on elemental sulfur as the major source of raw material.
There have been evolutions in the methods for the production of sulfuric acid, however, there are two major processes for its commercial production. There is the lead chamber process (no production after 1960s), and the contact process. Irrespective of the difference in the processes, the manufacture of sulfuric acid is generally based on the following principles:
-
The extraction of pure elemental sulfur from its ore.
-
The conversion of elemental sulfur into sulfur dioxide in the presence of oxygen.
-
The conversion of sulfur dioxide into sulfur trioxide.
-
The conversion of sulfur trioxide into sulfuric acid.
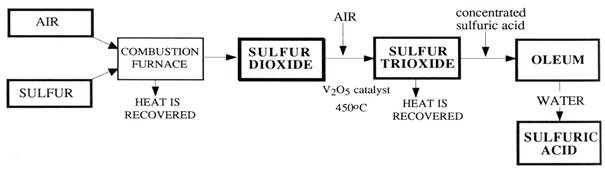
Burner
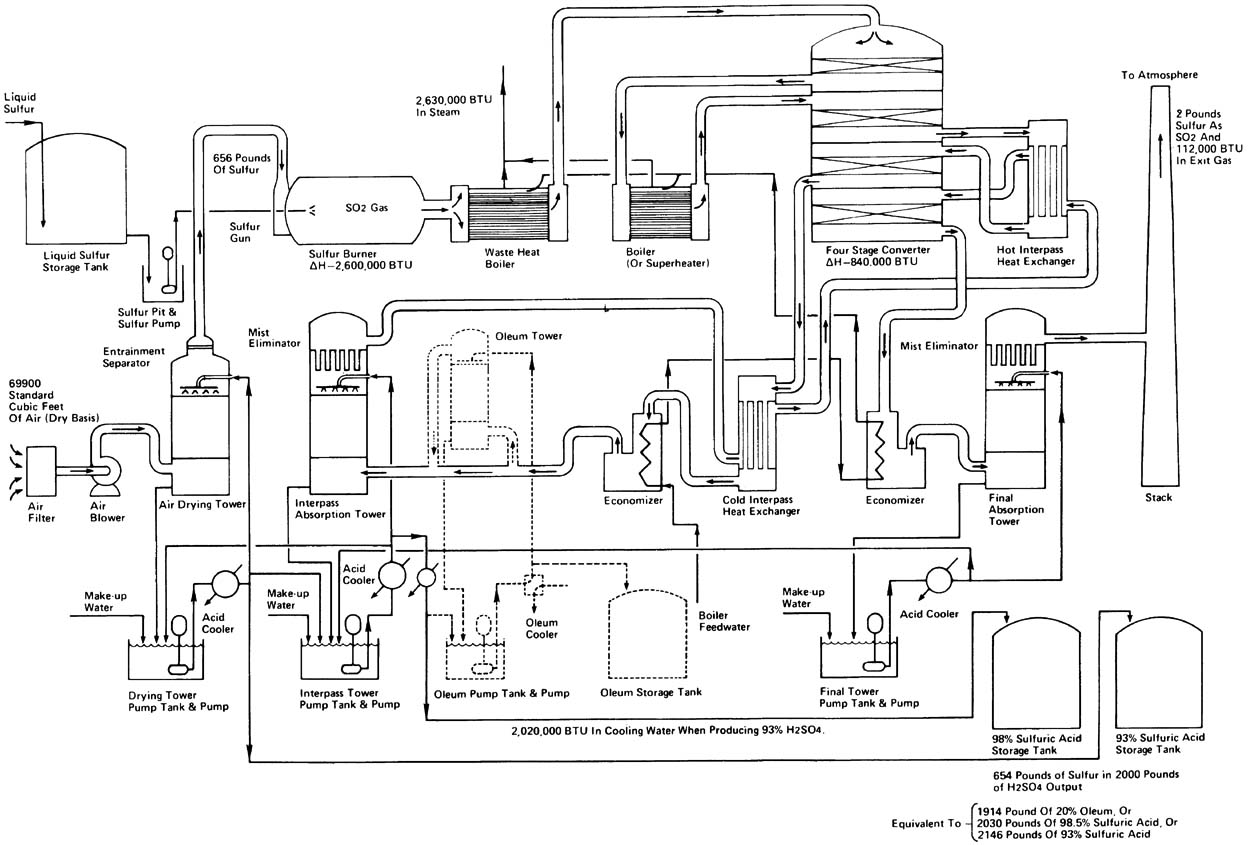
Sulfur is converted to sulfur dioxide by burning molten sulfur with dried air in a sulfur burner to yield a 1000-1200\(^\circ\)C gas stream containing 10–12 percent \(\ce{SO2}\). The burner is mounted at one end of a sulfur furnace, and the gas passed through a waste heat boiler at the other end. The gas temperature is reduced to 420-440\(^\circ\)C on leaving the boiler, which produces 40–60 bar steam. Modern sulfuric acid plants are generating 1.7 ton of steam per ton of sulfuric acid production.
Converter
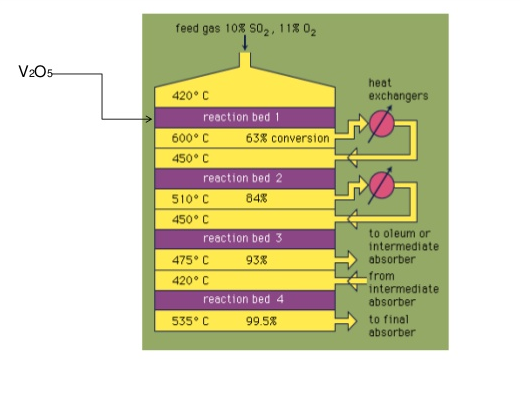
Being a reversible exothermic reaction, heat removal is very essential.
Absorption
\[\ce{SO3(g)} + \ce{H2O(l)} \rightarrow \ce{H2SO4(l)} \qquad \text{ $\Delta$H = -130.4 kJ/mol}\] Since the heat of reaction the above reaction between and producing sulfuric acid is exothermic, it will lead to the formation of acid mist. This mist is difficult to contain.
Instead of the above single step reaction, it is usually carried out in steps: forming oleum by absorption with concentrated sulfuric acid, followed by diluting it with water to get sulfuric acid.
Detailed Flowsheet
Sodium Carbonate
-
Sodium carbonate (\(\ce{NaCO3}\)) is also called as ‘soda ash’, or ‘washing soda’. Historically it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as “soda ash”.
-
Industrial production of sodium carbonate is from sodium chloride and limestone by the “Solvay process”.
-
In terms of its largest applications, sodium carbonate is used in the manufacture of glass, paper, rayon, soaps, and detergents. Sodium carbonate is a food additive used as an acidity regulator, anticaking agent, raising agent, and stabilizer.
Sodium carbonate is also used as a relatively strong base in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than \(\ce{NaOH}\) and far safer to handle. Its mildness especially recommends its use in domestic applications.
Sodium bicarbonate (\(\ce{NaHCO3}\)) or baking soda, also a component in fire extinguishers, is often generated from sodium carbonate. Although \(\ce{NaHCO3}\) is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some \(\ce{NaHCO3}\), making it more economical to react finished \(\ce{Na2CO3}\) with \(\ce{CO2}\):
\[\ce{Na2CO3} + \ce{CO2} + \ce{H2O} \rightarrow \ce{2NaHCO3}\]
Natural Occurence
“Trona”, trisodium hydrogendicarbonate dihydrate (\(\ce{Na3HCO3CO3.2H2O}\), also sodium sesquicarbonate dihydrate, \(\ce{Na2CO3.NaHCO3.2H2O}\)) is a non-marine evaporite mineral. It is mined in several areas of the USA and provides nearly all the domestic consumption of sodium carbonate. Large natural deposits found in 1938, such as the one near Green River, Wyoming, have made mining more economical than industrial production in North America.
Industrial Production
Leblanc process — was used from 1792, by using salt, sulfuric acid, limestone, and coal. In 1861, Solvay process was introduced. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.
Solvay process uses limestone (\(\ce{CaCO3}\)), coal / coke (C) / natural gas, salt (\(\ce{NaCl}\)) and air as the raw materials and uses ammonia as a cyclic reagent.
\[\ce{CaCO3} + \ce{C} + \ce{O2} + 2 \ce{NaCl} \rightarrow \ce{Na2CO3} + \ce{CO2} + \ce{CaCl2}\]
Solvay Process
Solvay process is based on the conversion of sodium chloride to sodium carbonate using ammonia and carbon dioxide.
\[\ce{NaCl} + \ce{NH3} + \ce{CO2} + \ce{H2O} \rightarrow \ce{NaHCO3} + \ce{NH4Cl}\] Ammoniated solution of salt is carbonated with \(\ce{CO2}\) from a coke-fired lime-kiln.
The precipitated sodium bicarbonate is converted to sodium carbonate by heating it, releasing water and carbon dioxide:
\[\ce{2NaHCO3} \rightarrow \ce{Na2CO3} + \ce{H2O} + \ce{CO2}\]
Ammonia is regenerated from the ammonium chloride byproduct by treating it with the milk of lime (calcium hydroxide) obtained from carbon dioxide generation:
\[\ce{2NH4Cl} + \ce{Ca(OH)2} \rightarrow \ce{2NH3} + \ce{CaCl2} + \ce{2H2O}\]
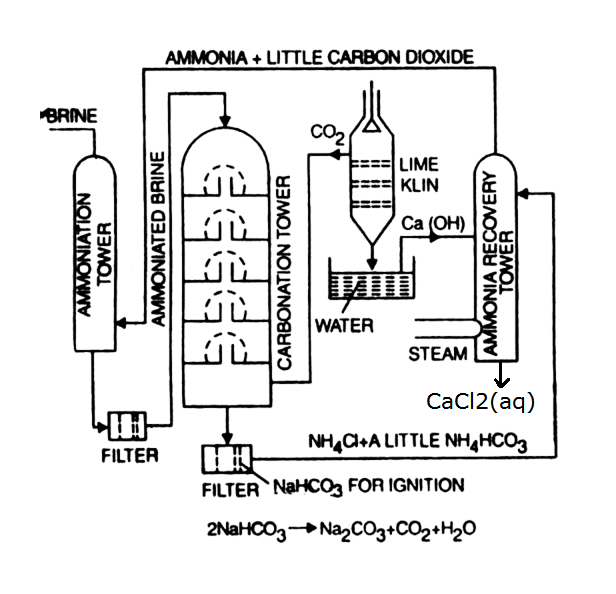
Recovery of Ammonia:
The lime-treated solution is fed to the top of the bubble-cap distillation unit. Steam is injected at the bottom, stripping out the ammonia down to a residual level of only 0.001 percent.
Calcination of \(\ce{NaHCO3}\):
The crude \(\ce{NaHCO3}\) is calcinated in dryers constructed with rotating seals and gas-tight feed and discharge mechanisms, to ensure the production of \(\ce{CO2}\) that is undiluted with air.
Quiz
Why is excess air used in sulfur oxidation than that stochiometrically required?
How is the heat released from sulfur to \(\ce{SO2}\) and \(\ce{SO3}\) reactions is recovered and used in the sulfuric acid industry?
What is the reason for having the \(\ce{SO2}\) to \(\ce{SO3}\) converter as a four-bed one instead of single-bed?
For the conversion of \(\ce{SO3}\) to \(\ce{H2SO4}\) what is the need for using concentrated sulfuric acid rather than water?
Why is sodium carbonate called as ‘soda ash’?
What is the role of \(\ce{NH3}\) in Solvay process?
How is the original Solvay process modified so as to overcome the pollution problem with \(\ce{CaCl2}\)?