Unit-1: Basic Concepts
Completion requirements
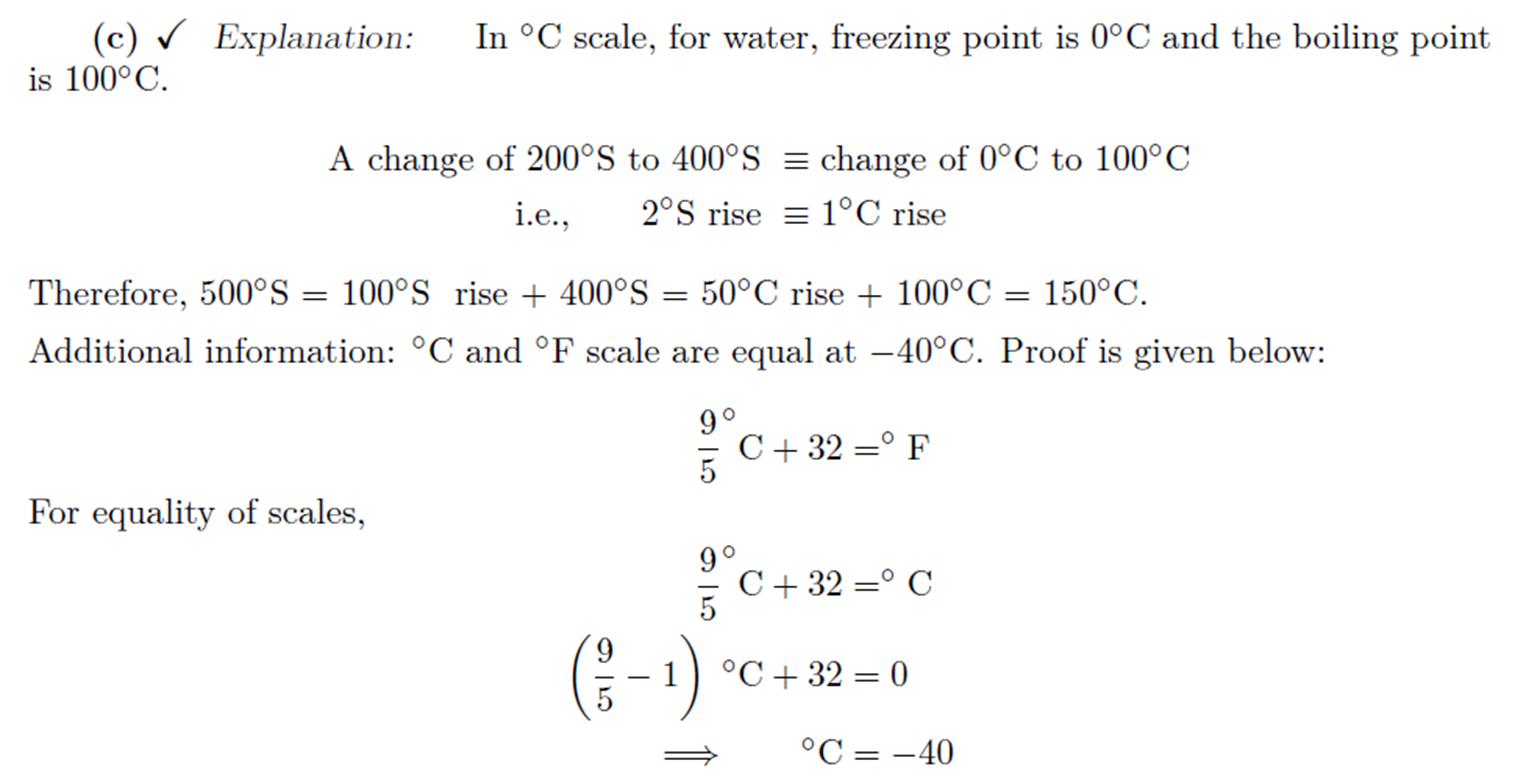
Zeroth Law of Thermodynamics
There
exists for every thermodynamic system in equilibrium a property called
temperature. Equality of temperature is a necessary and sufficient condition
for thermal equilibrium.
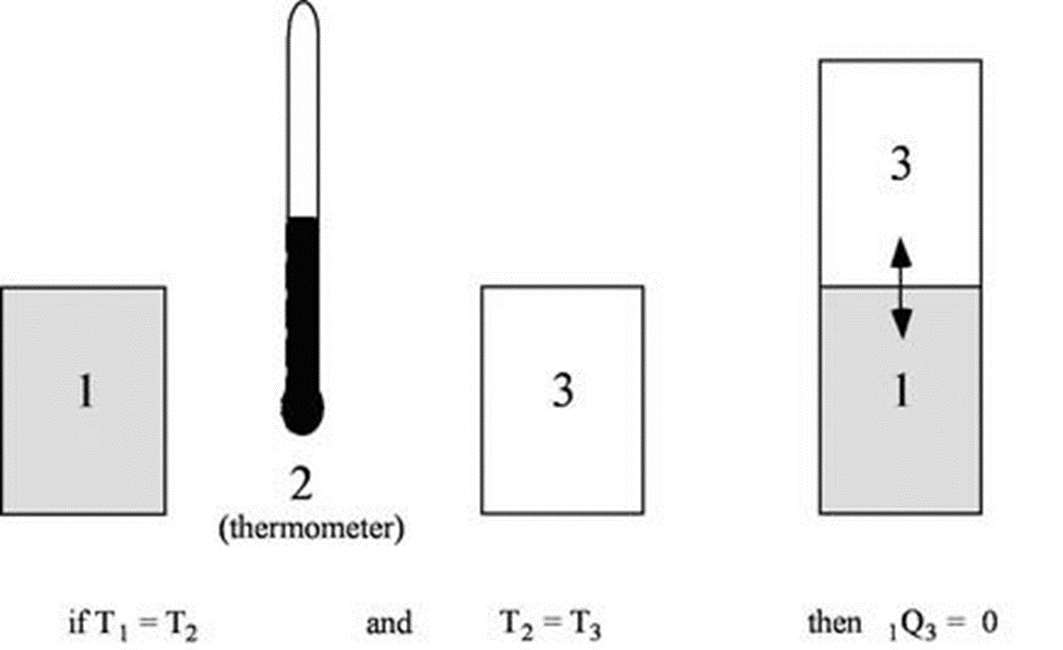
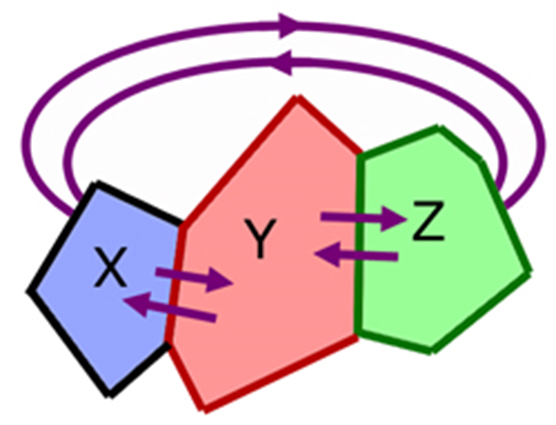
Consider three bodies, X, Y and Z.
Z is in thermal equilibrium with Y.
X is in thermal equilibrium with Y.
Then Z is in thermal equilibrium with X

To try to visualize this further, consider a hot cup of tea.
After about twelve hours, the saucer, the cup and the tea will all be at the same temperature.
The saucer is in equilibrium with the cup.
The cup
is in equilibrium with the tea.
Therefore the tea
is in equilibrium with the saucer.
They are each in thermal equilibrium with each other.