Unit-1: Basic Concepts
CP and CV
- We expect that CP will be bigger than CV for the simple reason that more heat will need to be added when heating at constant pressure than when heating at constant volume. This is because in the constant pressure heating additional energy will be expended on doing work on the atmosphere as the gas expands.
- It turns out that indeed CP is bigger than CV in practice
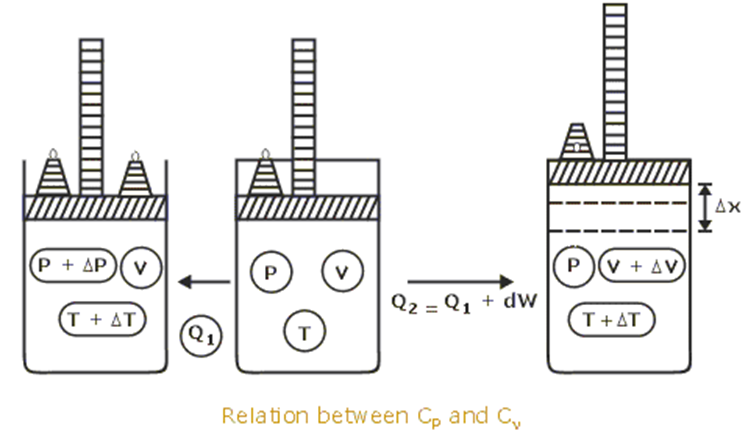
Figure: http://www.tutorvista.com/content/physics/physics-iii/heat-and-thermodynamics/mayers-formula.php
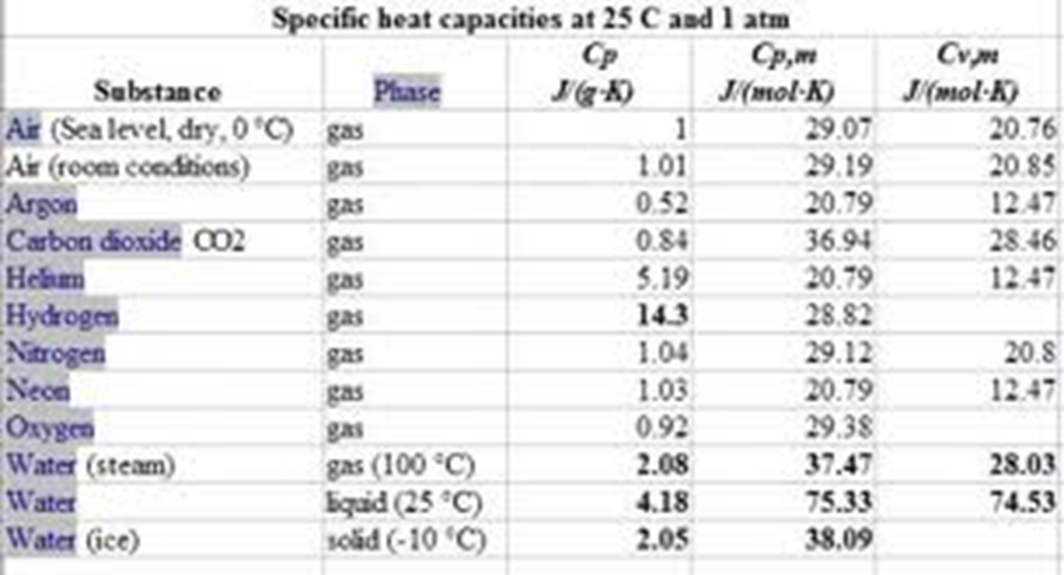
Specific heat values: Notice, however, that, because water has a low molecular weight (molar mass), water has the largest specific heat capacity of any common liquid or solid. (The specific heat capacities of gaseous H2 and He are, unsurprisingly, larger still. A kilogram of hydrogen is an enormous number of molecules, so it takes a lot of heat to warm them all up.)
For liquids In general, Cp – Cv is so small in comparison with gases; but there are cases where Cp – Cv is higher than R.
Ref: Norman O. Smith, J. Chem. Educ., 1965, 42 (12), p 654
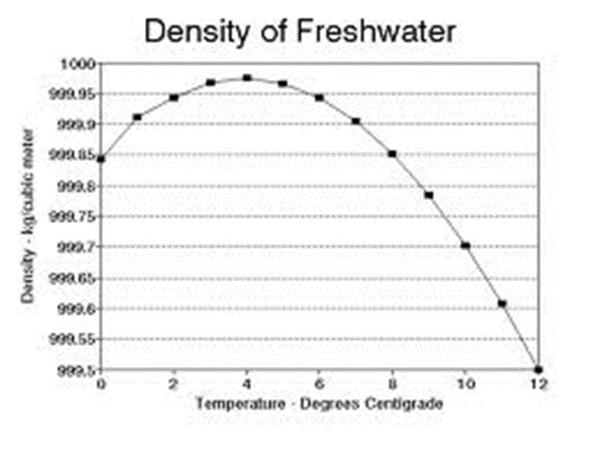
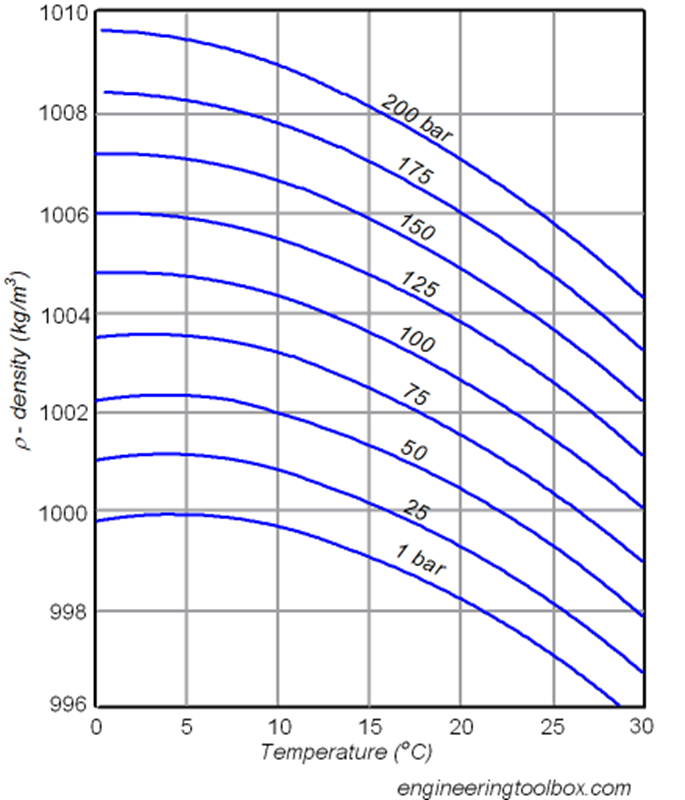
The heat capacity CP is less than CV for H2O(l) near 4°C. Explain this result.
CP < CV is valid if V decreases with T at constant P.
This unusual behavior occurs because the density of water increases with temperature in this range of 0 to 4oC. Therefore, work is done by the surroundings on the system as water is heated at constant P between 0 ºC and 4 ºC.