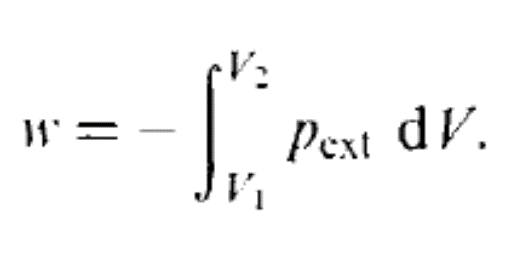Unit-1: Basic Concepts
Completion requirements
Quasi-static process
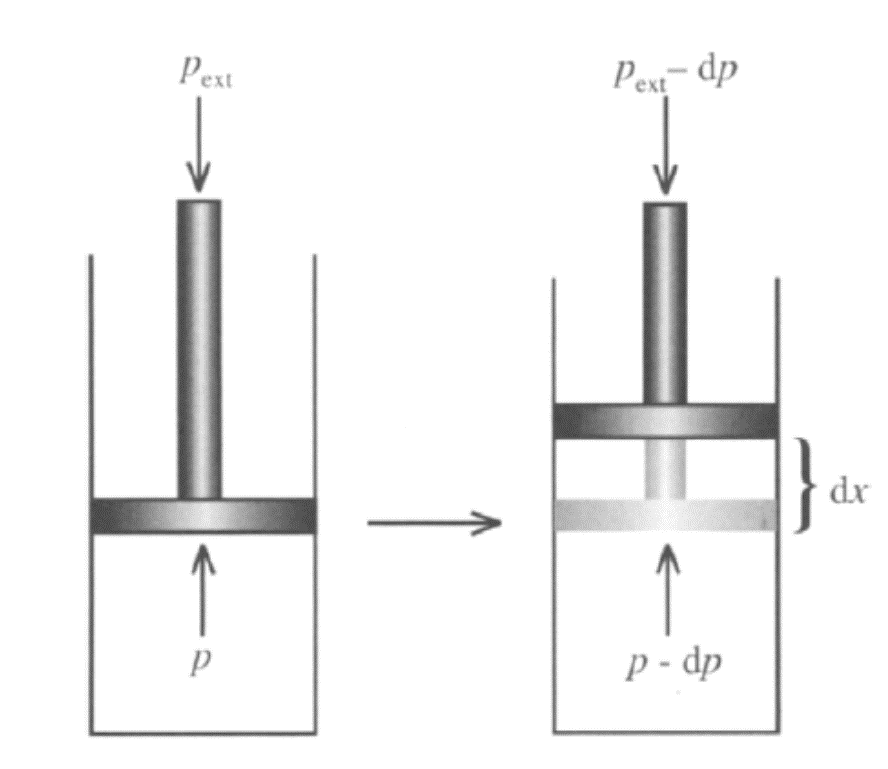
A quasistatic process ensures that the system will go through a sequence of states that are infinitesimally close to equilibrium, in which case the process is typically reversible. Any reversible process is necessarily a quasistatic one. However, some quasistatic processes are irreversible, if there is heat flowing (in or out of the system) or if entropy is being created in some other way.
An example of a quasistatic process that is not reversible is a compression against a system with a piston subject to friction — Although the system is always in thermal equilibrium, the friction ensures the generation of dissipative entropy, which directly goes against the definition of reversible.
The hallmark of a quasistatic process is that the system is at any point along the process very close to equilibrium and can be described with the equilibrium independent variables.
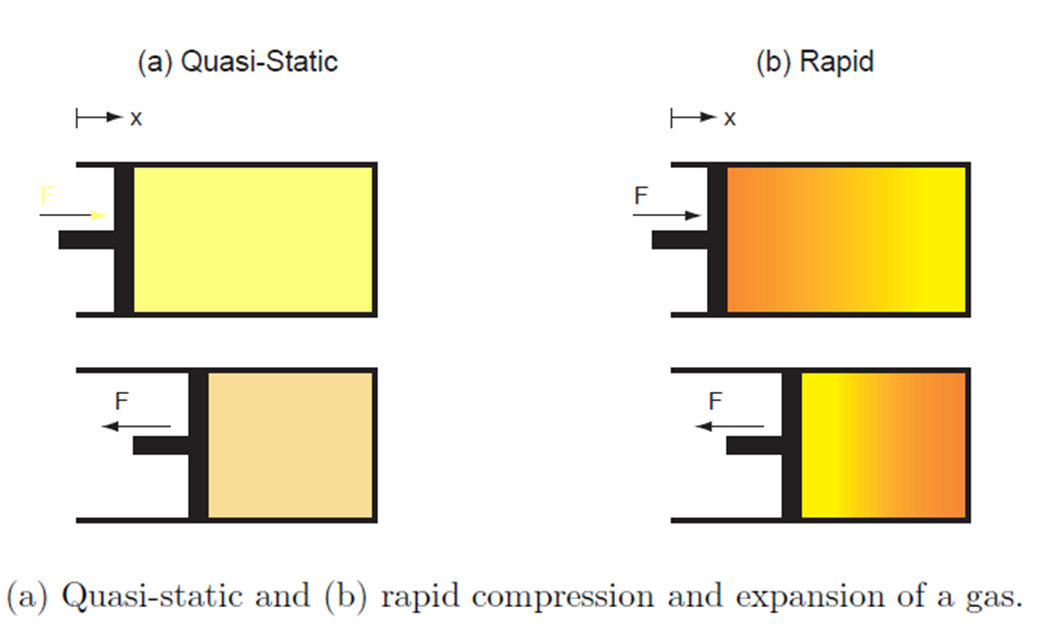
Actual processes are not quasi-static. To change the pressure of a gas, we can move a piston inside the enclosure. The gas near the piston is acted upon by greater force as compared to the gas away from the piston. The pressure of the gas may not be uniform everywhere while the piston is moving. However, we can move the piston very slowly to make the process as close to quasi-static as we wish. Thus, in a quasi-static process, all changes take place extremely slowly.