Unit-3: First Law of Thermodynamics
Completion requirements
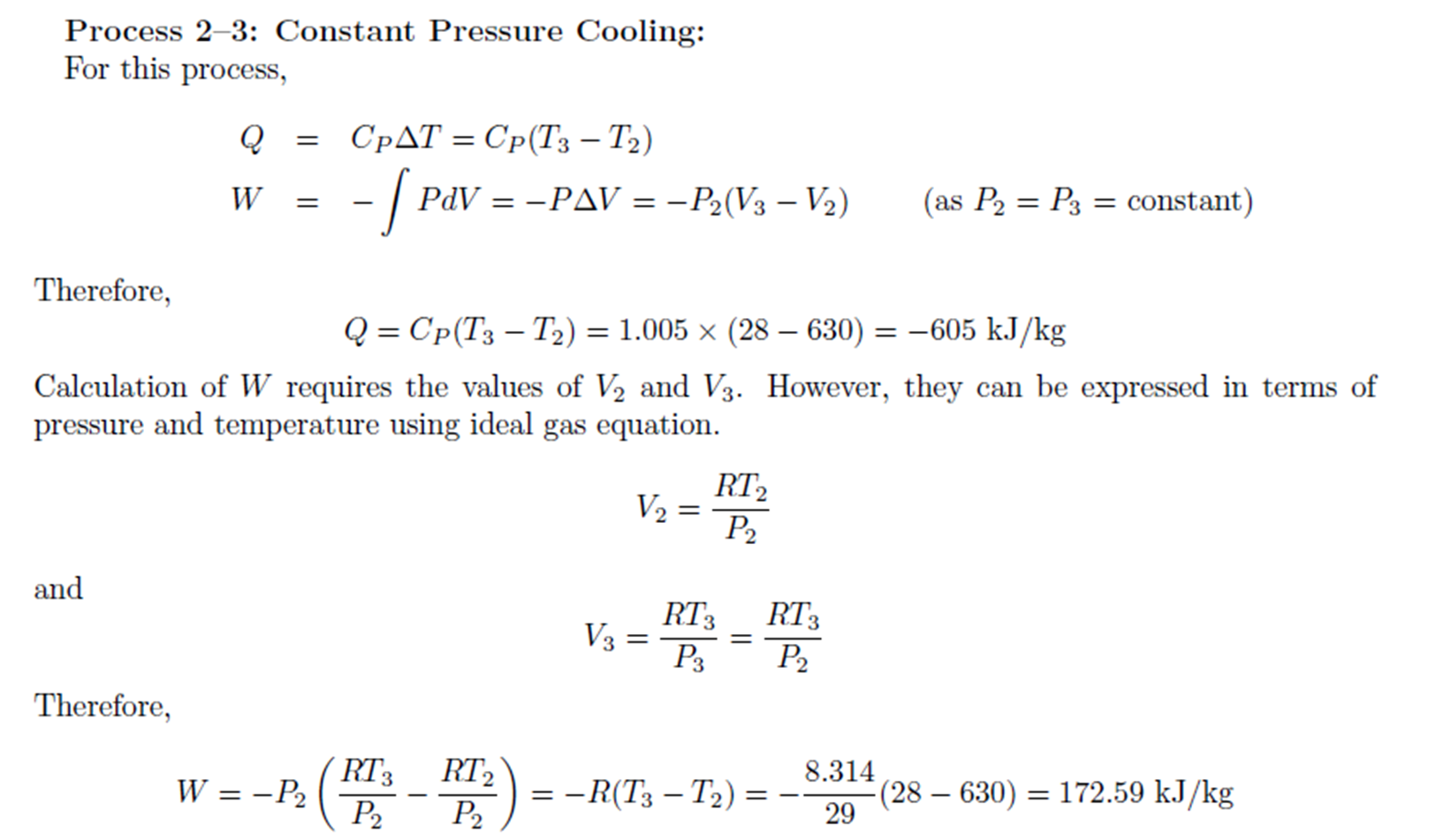
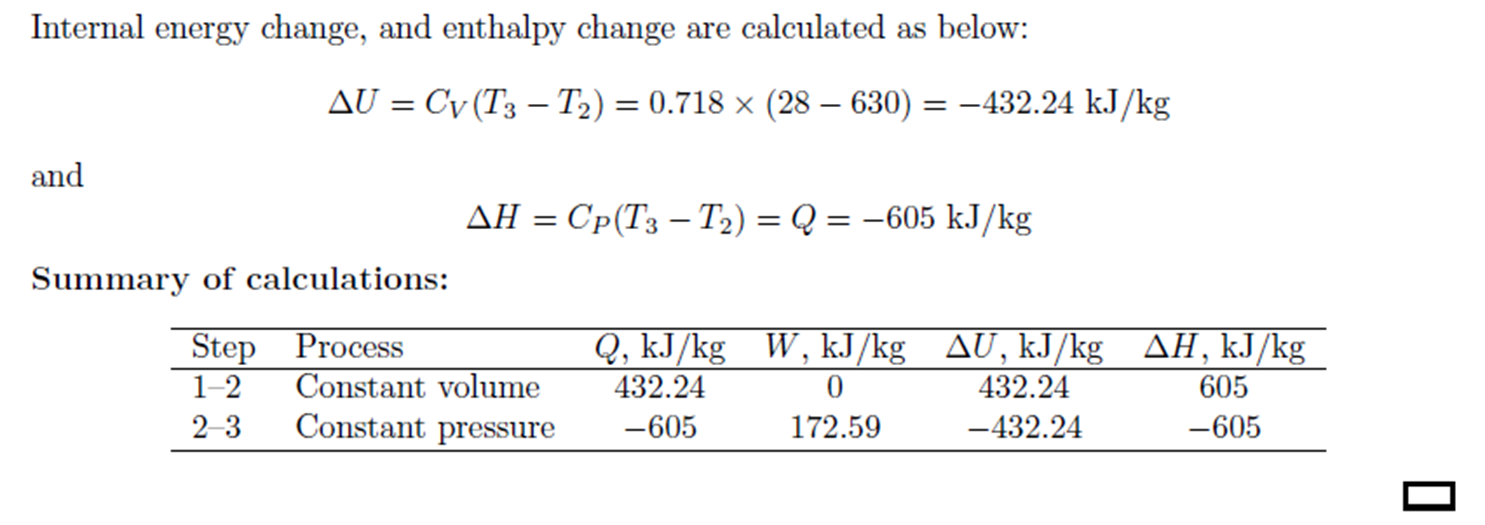
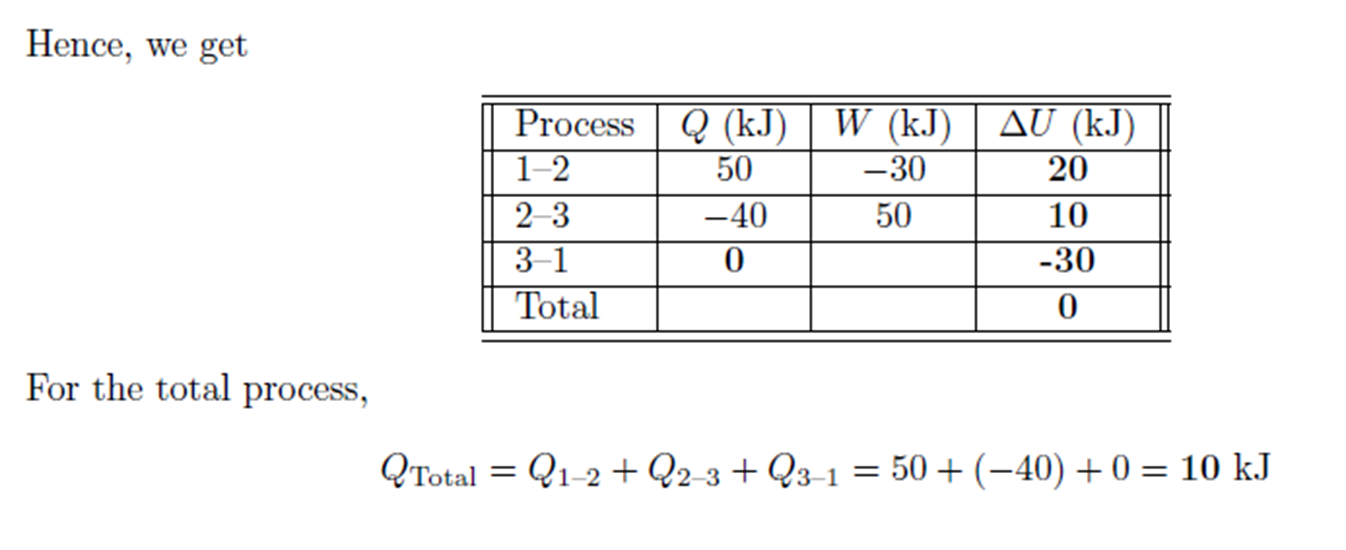
Example: Constant Volume Heating, Constant Pressure Cooling
Example:
Constant Volume Heating, Constant Pressure Cooling
Air is compressed from 2 atm absolute and 28oC
to 6 atm absolute and 28oC
by heating at constant volume followed by cooling at constant pressure.
Calculate the heat and work requirements and \( \Delta \)U and \( \Delta \)H
of the air. CV
of air = 0.718 kJ/kg.oC