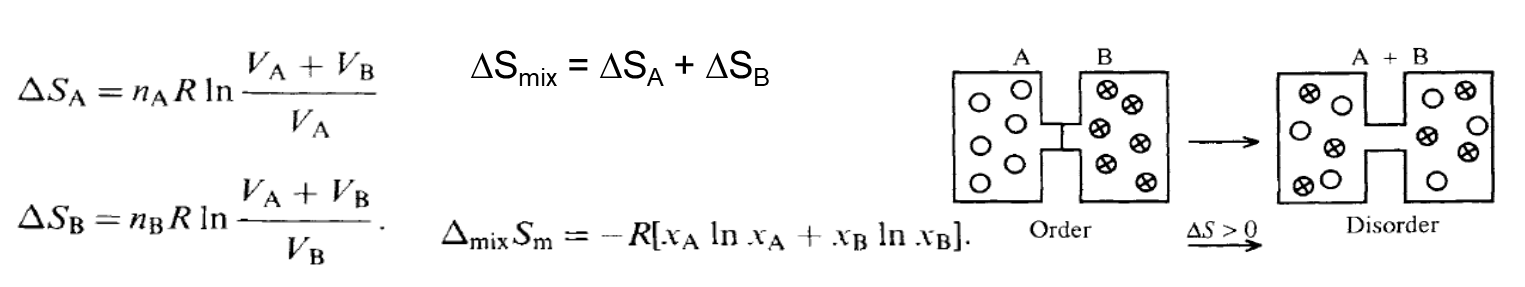Unit-4: Second Law of Thermodynamics
Completion requirements
Entropy Change of Mixing
Entropy
Change of Mixing
- Consider the process, where nA moles of ideal gas A are confined in a bulb of volume VA at a pressure P and temperature T. This bulb is separated by a valve or stopcock from bulb B of volume VB that contains nB moles of ideal gas B at the same pressure P and temperature T. When the stopcock is opened, the gas molecules mix spontaneously and irreversibly, and an increase in entropy \( \Delta \)Smix occurs.
- The entropy change can be calculated by recognizing that the gas molecules do not interact, since the gases are ideal. \( \Delta \)Smix is then simply the sum of \( \Delta \)SA, the entropy change for the expansion of gas A from VA to (VA + VB) and \( \Delta \)SB, the entropy change for the expansion of gas B from VB to (VA + VB). That is,