2. Material Balance Calculations
Conversion
-
Conversion: Refer to Fig.(2).
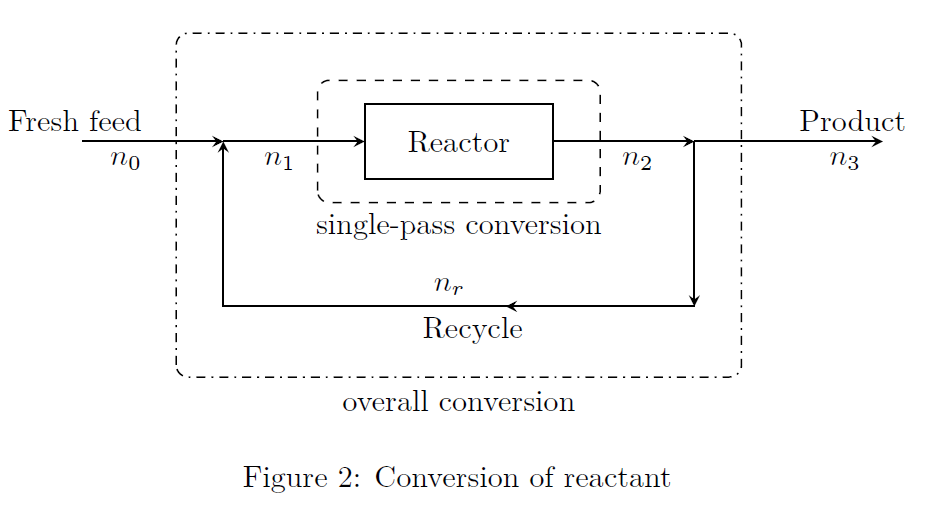
\[\begin{align*} \text{Overall conversion} &= \frac{\text{reactant input to process}-\text{reactant output from process}}{\text{reactant input to process}} \tag*{(4)} \\ &= \frac{n_0-n_3}{n_0} \\ \text{Single-pass conversion} &= \frac{\text{reactant input to reactor}-\text{reactant output from reactor}}{\text{reactant input to reactor}} \tag*{(5)} \\ &= \frac{n_1-n_2}{n_1} \end{align*}\]
-
Conversion calculation is based on the limiting reactant. Single-pass conversion is also called as once-through conversion, or conversion per pass. Because of the recycle, the overall conversion—which is based on the fresh feed, will be higher than the conversion per pass—which is based on the mixed feed to the reactor.
-
Yield and Selectivity
-
Yield: The actual amount of products formed relative to what is actually expected from the reaction. \[\text{% Yield} = \frac{\text{actual amount of product formed}} {\text{theoretical amount of product expected}} \times 100\]
-
Selectivity: The ratio of the moles of the desired product produced to the moles of undesired product (by-product). \[\text{Selectivity} = \frac{\text{moles of desired product formed}}{\text{moles of undesired product formed}}\] For example, methanol (\(\ce{CH3OH}\)) can be converted into ethylene (\(\ce{C2H4}\)) and propylene (\(\ce{C3H6}\)) by the reactions: \[\begin{aligned} \ce{2CH3OH} &\rightarrow \ce{C2H4} + \ce{2H2O} \\ \ce{3CH3OH} &\rightarrow \ce{C3H6} + \ce{3H2O}\end{aligned}\] If the desired product is ethylene, then the selectivity is computed as: \[\text{Selectivity} = \frac{\text{moles of ethylene formed}}{\text{moles of propylene formed}}\]
-
-
Recovery \[\text{% Recovery} = \frac{\text{desired product in output stream}} {\text{desired product in input stream}} \times 100\]
