1. Introduction
1.2 Feasibility of a Chemical Reaction
Spontaneous Process
-
A process which can proceed in a given direction without the need of outside energy is called a spontaneous process. e.g., A spontaneous chemical reaction.
-
Enthalpy and entropy are the driving forces responsible for the spontaneity of chemical reactions.
-
Some reactions are spontaneous because they liberate energy in the form of heat (\(\Delta H<0\)).
-
Some reactions are spontaneous since they lead to an increase in the disorder of the system (\(\Delta S>0\)).
If one driving force is favorable for the reaction, and the other is not, then the relative importance of the driving forces, i.e., enthalpy and entropy, is to be determined. The thermodynamic function, known as the Gibb’s free energy (\(G\), simply called ‘free energy’) is defined to determine the relative importance of the two driving forces behind a particular reaction. \(\Delta G\) is defined, at constant \(T\), as \[\Delta G = \Delta H - T\Delta S\]
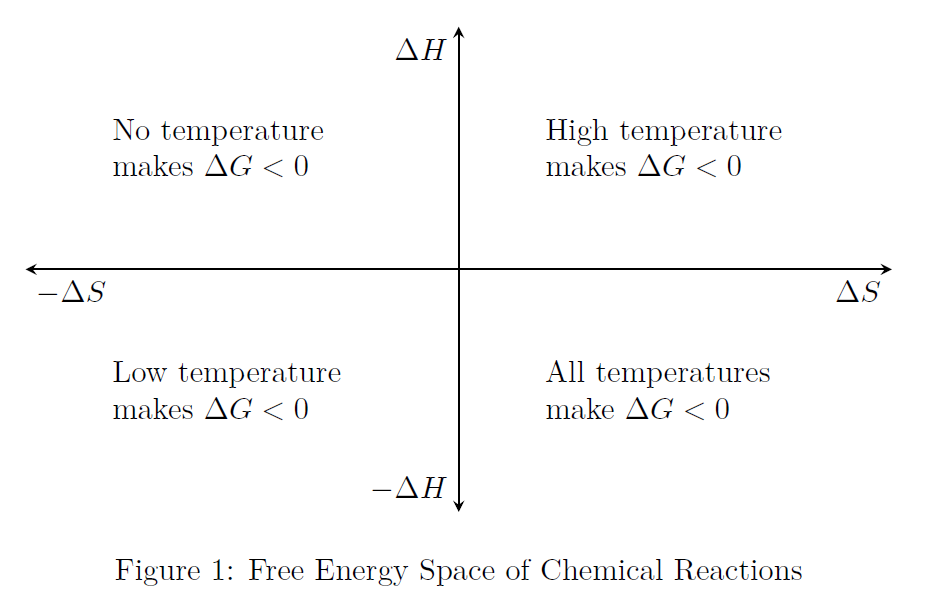 Refer to Fig.(1) which demonstrates
the choice of temperature to be made in order to make the reaction feasible
Refer to Fig.(1) which demonstrates
the choice of temperature to be made in order to make the reaction feasible-
\(\Delta G\) is a man-made property.
-
\(\Delta G\) cannot be measured.
\[\Delta G = \Delta G^\circ + RT\ln K\] At equilibrium, \(\Delta G\) is zero. Hence, \[\Delta G^\circ = -RT\ln K\]
-
-
The free energy of a reaction at any moment in time is a measure of the energy available to do work.
