1. Modes of Heat Transfer
Conduction
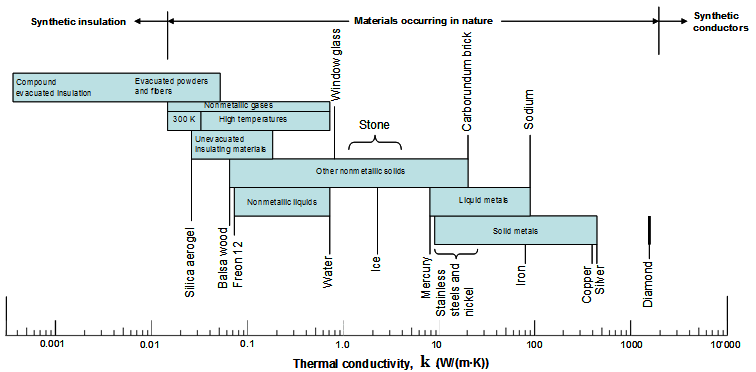
Thermal Conductivity
The thermal conductivity is the rate of thermal energy transfer per unit area and per unit temperature gradient.
-
Thermal conductivities of solids at room temperature vary from 0.1 W/(m.K) for good insulators (e.g. asbestos) up to 400 W/(m.K) for good conductors (e.g. silver).
-
The conductivity changes mildly with temperature except at very low temperatures where it can acquire very large values. For instance, pure copper at 10 K has a conductivity of about 20,000 W/(m.K).
-
Pure crystals and metals have the highest thermal conductivities, and gases and insulating materials the lowest.
-
The thermal conductivity of a substance is normally highest in the solid phase and lowest in the gas phase.
-
Specific heat \(C_P\) is a measure of a material’s ability to store thermal energy. For example \(C_P = 4.184\) kJ/kg.\(^\circ\)C for water and \(C_P = 0.45\) kJ/kg.\(^\circ\)C for iron at room temperature, which indicates that water can store almost 10 times the energy that iron can per unit mass.
-
Likewise thermal conductivity \(k\) is a measure of a material’s ability to conduct heat. For example, \(k = 0.608\) W/m.\(^\circ\)C for water and \(k = 80.2\) W/m.\(^\circ\)C for iron at room temperature, which indicates that iron conducts heat more than 100 times faster than water can.
Thus water is a poor heat conductor relative to iron, although water is an excellent medium to store thermal energy.
