Need for Multiple Stages
Completion requirements
Solubility
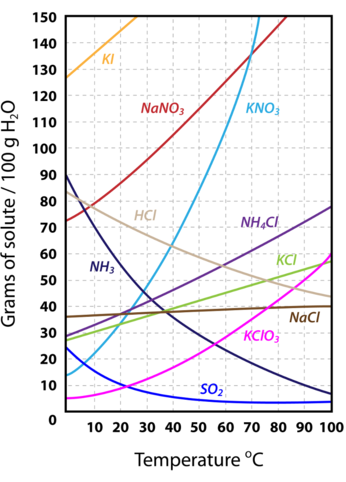
Source: www.ck12.org/book/ck-12-chemistry-concepts---intermediate/r25/section/16.4/
-
Solubility is the amount of solute that can dissolve in a given amount of solvent at a given temperature.
-
Solubility curves can be used to determine if a given solution is saturated or unsaturated.
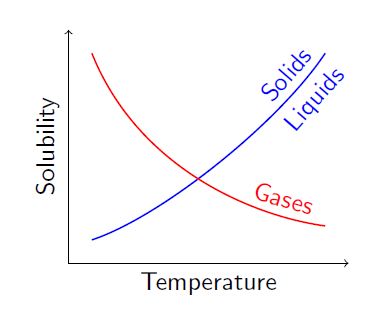
With solid or liquid solutes, increasing the temperature increases its solubility. For example, solubility of sugar is more in hot water than in cold water.
If the solute is a gas, increasing the temperature decreases its solubility. For example, oxygen’s solubility is lesser in warm water than in cold water.
