Instant Notes: 2. Interpretation of Batch Reactor Data
Completion requirements
(a) Irreversible Unimolecular-type First-order Reactions
\[A \stackrel{k}{\rightarrow} R\] \[\begin{align*} -r_A = -\frac{dC_A}{dt} &= kC_A \nonumber \\ -\ln\frac{C_A}{C_{A0}} &= kt \tag*{(1)} \\ \text{Since, $C_A=C_{A0}(1-X_A)$} \\ -\ln(1-X_A) &= kt \tag*{(2)}\end{align*}\] The above two equations are plotted in Fig.(4).
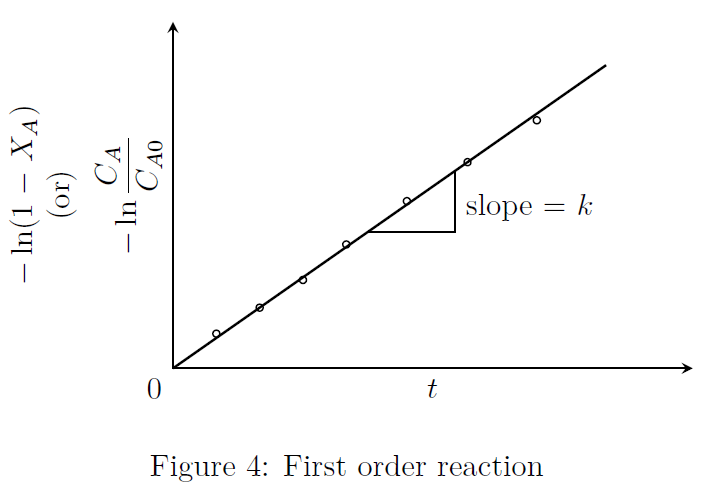
Half-life (\(t_{1/2}\)): \(C_A=C_{A0}/2\) for \(t=t_{1/2}\). Substituting these in Eqn.(1), we get \[\begin{align*} -\ln\left(\frac{C_{A0}/2}{C_{A0}}\right) &= k\, t_{1/2} \nonumber \\ \Longrightarrow \quad t_{1/2} &= \frac{0.693}{k} \tag*{(3)}\end{align*}\]
