Instant Notes: 2. Interpretation of Batch Reactor Data
(e) Irreversible Parallel Reactions
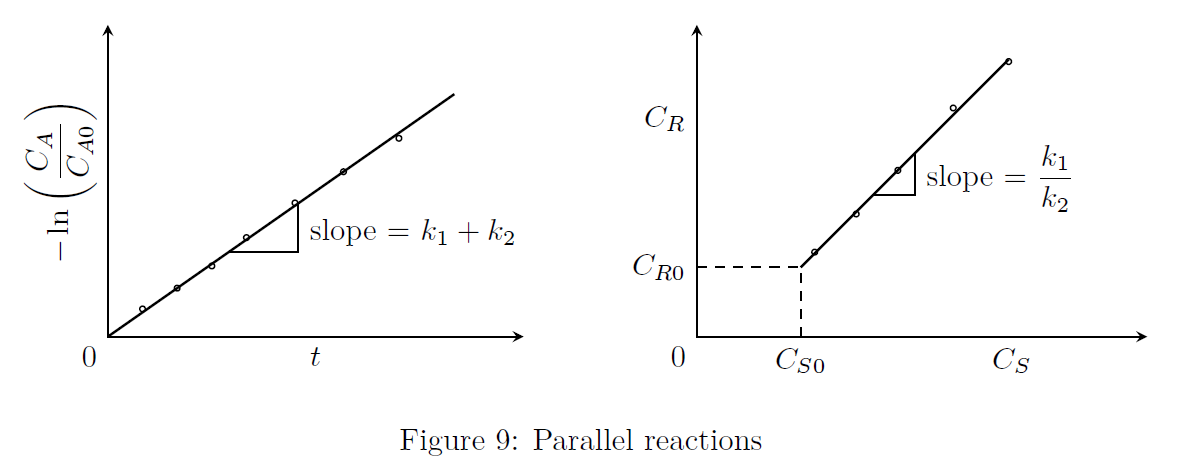
Consider the following elementary reactions. \[\begin{align*} A \stackrel{k_1}{\rightarrow} R \qquad & r_R &= \frac{dC_R}{dt} = k_1C_A \\ A \stackrel{k_2}{\rightarrow} S \qquad & r_S &= \frac{dC_S}{dt} = k_2C_A \end{align*}\] \[\begin{align*} -r_A &= -\frac{dC_A}{dt} = (k_1+k_2)C_A = r_R + r_S \\ \text{Integrating,} \\ -\ln\left(\frac{C_A}{C_{A0}}\right) &= (k_1+k_2)t \end{align*}\] Also, \[\begin{align*} \frac{r_R}{r_S} &= \frac{dC_R}{dC_S} = \frac{k_1C_A}{k_2C_A} = \frac{k_1}{k_2} \\ \text{Integrating,} \\ \int_{C_{R0}}^{C_R}dC_R &= \frac{k_1}{k_2}\int_{C_{S0}}^{C_S}dC_S \\ \Longrightarrow \quad \frac{C_R-C_{R0}}{C_S-C_{S0}} &= \frac{k_1}{k_2}\end{align*}\] The graphs as shown in Fig.(9) may be used to evaluate the values of \(k_1\) and \(k_2\).