Evaporation
An evaporator is fed continuously with 25 kg/hr of a solution which contains 10% NaCl, 10% NaOH and 80% H2O. During evaporation, H2O is removed from the solution and NaCl precipitates as crystals which is settled and removed. The concentrated liquor leaving the evaporator contains 50% NaOH, 2% NaCl and 48% H2O. Calculate
(i)Weight of salt precipitated per hour. (6)
(ii) Weight of concentrated liquor leaving per hour. (6)
Calculations:
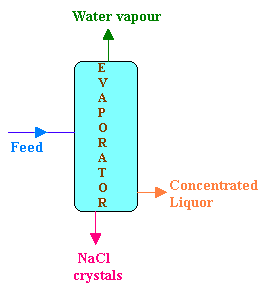
Data:
Flow rate of feed = 25 kg/hr
Composition of feed (F):
NaCl 10%
NaOH 10%
H2O 80%
Composition of concentrated liquor (P):
NaCl 2%
NaOH 48%
H2O 50%
Balance for NaOH:
NaOH entering the in feed = NaOH leaving in concentrated liquor
i.e., 0.1 x 25 = 0.50 P
Therefore, the mass flow rate of concentrated liquor (P) = 0.1 x 25 / 0.50 = 5 kg/hr
Balance for NaCl:
NaCl entering in the feed = 25 x 0.1 = 2.5 kg/hr
The entering NaCl is coming out as crystals and in the concentrated liquor.
NaCl leaving in the concentrated liquor = 0.02 P = 0.02 x 5 = 0.1 kg/hr
Therefore,
NaCl precipitated = 2.5 - 0.1 = 2.4 kg/hr
