T-x-y Diagram of Ideal Solution
Completion requirements
From the following vapor pressure data, construct the temperature - composition diagram at 1 atm, for the system benzene-toluene, assuming ideal solution behavior.
Temperature oC | Vapor pressure, mm Hg | |
Benzene | Toluene | |
80 | 760 | 300 |
92 | 1078 | 432 |
100 | 1344 | 559 |
110.4 | 1748 | 760 |
Calculations:
Mole fraction in liquid phase (x) is given by,
x = (pt - pB)/(pA - pB)
The corresponding equilibrium mole fraction in vapor phase (y) is,
y = pAx/pt
Using these equations, for the various temperatures given, the following data for x and y are obtained, for the total pressure of 1 atm
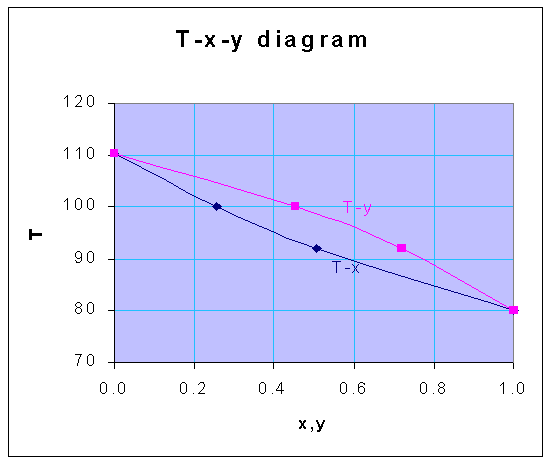
| T | x | y |
| 80 | 1.000 | 1.000 |
| 92 | 0.508 | 0.720 |
| 100 | 0.256 | 0.453 |
| 110.4 | 0.000 | 0.000 |
From the above-calculated data, the following T-x-y diagram is drawn.
Last modified: Saturday, 23 March 2024, 7:22 PM
