Activation Energy
Completion requirements
The decomposition of NO2 follows a second order rate equation. Data at different temperatures are as follows:
T (K) 592 603 627 651.5 656 k (cm3/gmol.sec) 522 755 1700 4020 5030
Compute the energy of activation Energy from the data. The reaction is 2NO2 → 2NO + O2
Calculations:
Activation energy is found from the Arrhenius' relation
k = ko e-E/RT
i.e.,
ln k vs. 1/T is a straight line with a slope of -E/R
where, E is the activation energy; and
R is the universal gas constant.
T (K) | 592 | 603 | 627 | 651.5 | 656 |
k (cm3/gmol.sec) | 522 | 755 | 1700 | 4020 | 5030 |
1/T (oK-1) | 0.001689 | 0.001658 | 0.001595 | 0.001535 | 0.001524 |
ln k | 6.257668 | 6.626718 | 7.438384 | 8.299037 | 8.523175 |
From the above data, the following graph is drawn, and the slope is = -13622
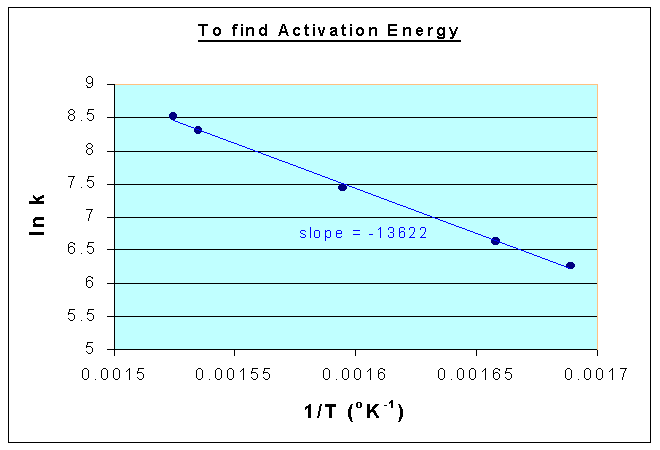
Therefore,
E/R = 13622
E = 13622 x 8314 = 113253.3 kJ/kmol
Last modified: Saturday, 23 March 2024, 8:08 PM
