Fluidization
Fluidization refers to those gas-solids and liquid-solids system in which the solid phase is subjected to behave more or less like a fluid by the upwelling current of gas or liquid stream moving through the bed of solid particles.
Fluidized bed combustion and catalytic cracking of heavy crude-oil fractions of petroleum are the two good examples of fluidization.
Fluidization starts at a point when the bed pressure drop exactly balances the net downward forces (gravity minus buoyancy forces) on the bed packing, so
Dp/L = (1-e)(rs - r)g à 1
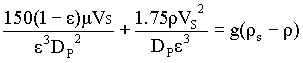 à 2
à 2
substituting for Dp/L from Ergun's equation,
In most industrial applications involving fluidized beds, the particle diameter is small, and VS also small. In these cases, the second term of the above equation is negligible compared to the first, so that
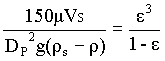 à 3
à 3
For a given bed the above equation can be used for both the unexpanded and the expanded state.
Pressure Drop Behavior of Fluidized beds:
As VS increases, e may increase and hold Dp constant (L will also increase but its effect is much less than the effect of change in e. See all the above equations). Thus the experimental result for such a test is shown in figure.
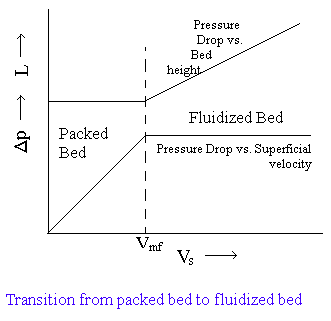
For velocities less than the minimum fluidization velocity Vmf, the bed behaves as a packed bed. However as the velocity is increased past Vmf, not only does the bed expand (L increases), but also the particles move apart, and e also increases to keep the Dp constant.
As the velocity is further increased, the bed become more and more expanded, and the solid content becomes more and more dilute. Finally, the velocity becomes as large as terminal settling velocity Vt of the individual particles, so the particles are blown out of the system. Thus the velocity range for which a fluidized bed can exist is from Vmf (can be calculated from equn.2) to Vt.
The equations derived for minimum fluidization velocity apply to liquids as well as gases, but beyond the minimum fluidization velocity Vmf, the appearance of beds with liquids or gases is quite different.
When fluidizing sand with water, the particles move further apart and their motion becomes more vigorous as the velocity is increased, but the bed density at a given velocity is same in all sections of the bed. This is called particulate fluidization and is characterized by a large but uniform expansion of the bed at high velocities.
Beds of solids fluidized with air usually exhibit what is called aggregative or bubbling fluidization. At superficial velocities much greater than Vmf most of the gas passes through the bed as bubbles or voids which are almost free of solids, and only a small fraction of the gas flows in the channels between the particles.
Advantages and Disadvantages of fluidization:
The chief advantage of fluidization are that the solid is vigorously agitated by the fluid passing through the bed, and the mixing of the solid ensures that there are practically no temperature gradients in the bed even with quite exothermic or endothermic reactions.
Disadvantages:
- The main disadvantage of gas-solid fluidization is the uneven contacting of gas and solid.
- Erosion of vessel internals
- Attrition of solids. Because of attrition, the size of the solid particles is getting reduced and possibility for entrapment of solid particles with the fluid are more.
