Thermodynamics - October 1997
5HC - Chemical Engineering Thermodynamics - October 1997
Part A (20 x 2 = 40 Marks)
- A centrifugal fan forms _______ system
- Zeroth law of thermodynamics defines _______
- The ratio of specific heat of a gas at constant pressure and at constant volume always varies with _______
- Internal energy of ideal gas is a function of _______ alone
- Carnot cycle comprises _______ isothermal process and two _______ process
- A heat engine is supplied with 300 kJ/sec of heat at 600 K and heat rejection takes place at 300 K. Heat rejected is 100 kJ/sec. The data refers to _______ cycle
- Value of extensive properties of a system depends on _______ of the system
- Heat and work are _______ functions
- For a higher value of polytropic index the compressor work for a given pressure ratio is _______
- Enthalpy of a gas depends on _______
- Inter-cooling in an air compressor helps in _______
- The function of providing clearance volume in a reciprocating air compressor is _______
- One ton of refrigeration is _______
- The refrigerant used in household refrigerator is _______
- The entropy of the universe _______
- The application of Gibbs Duhem equation is _______
- The compressibility factor of an ideal gas is _______
- What is fugacity coefficient?
- Define Activity
- Define equilibrium constant
- (a) What is the scope and limitations of thermodynamics? (6)
- (a) Explain compressibility factor (5)
- (a) Show that CP - CV = R for an ideal gas
- (a) Derive the coexistence equation (8)
- (a) What is the effect of pressure on equilibrium constant? (6)
(b) Explain
(i) System and Surroundings (3)
(ii) Macroscopic and Microscopic aspects (3)
Or
(c) Explain the work required in the case of Isothermal and Adiabatic process (8)
(d) Calculate the work done when 65.38 gram of zinc dissolves in HCl (i) in a open beaker (ii) a closed beaker at 300 K (4)
(b) Derive van der Waal's equation of stae (7)
Or
(c) Show that in multistage compression for minimum work, the inter-stage pressure is the geometric mean of the initial and final pressures (8)
(d) Methane is to be compressed in an adiabatic compressor with inter-stage cooling from an initial pressure of 1.03 x 105 N/m2 to 201.06 x 105 N/m2. If the number of stages in the compressor is 4, what would be the compression ratio for optimal operation? (4)
(b) Show that 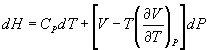
Or
(c) What is chemical potential? (3)
(d) Explain (i) Helmholtz free energy (ii) Gibbs free energy (iii) Joule Thomson Coefficient (3 x 3 = 9)
(b) For a binary mixture of 'A' and 'B' activity coefficient data for 'A' are available over the entire composition range, while one data is available for 'B'. Show how you would determine activity coefficient for 'B' over the entire range of composition (4)
Or
(c) Explain equilibrium curves and B.P. diagrams (6)
(d) An experimental determination of a VLE state for the ethanol, toluene system gave the following results:
Standard V.P. at 45oC, Ethanol = 173 mm Hg, Toluene = 75.4 mm Hg, x1 = 0.3; y1 = 0.634; PT = 183 mm Hg. Calculate (i) the liquid phase activity coefficient (ii) does the liquid phase exhibit positive or negative deviation from the ideal solution behavior? (6)
(b) Compute the equilibrium constant for the water gas reaction at 540oC (6)
CO + H2O à CO2 + H2
Data:
| DHf cal/gmol |
CO | -26,420 |
H2O | -57,800 |
CO2 | -94,050 |
H2 | 0 |
Or
(c) For the reaction SO2 + 1/2 O2 à SO3 in equilibrium at 900 K, what pressure is required for a 90% conversion of SO2 if the initial mixture is equimolar in the reactants. Take Ka = 1.3 at 900 K.
