Energy Balance Calculations
Section outline
-
Energy balance calculations for processes with no chemical reaction, specific heat, latent heat. Energy balance calculations for processes with single / multiple reactions. Heat of reaction from heat of formation, heat of combustion. Adiabatic reaction temperature.
-
Theory with Examples Page
-
Steam Mixing Page
-
Adiabatic Mixing of Steam Page
-
Temperature vs Time Curve for Heating of Water Page
-
Heat of Formation from Heat of Combustion Data Page
-
Heat of Reaction from Heat of Combustion Page
-
Heat of Reaction at Constant Volume Page
-
Amount of Heat Transferred to Reactor Page
-
Heat Released from Reaction Page
-
Adiabatic Flame Temperature of Natural Gas Combustion PageA natural gas containing 82 mole% \(\ce{CH4}\) and the balance \(\ce{C2H6}\) is burned with 20% excess air in a boiler furnace. The fuel gas enters the furnace at 298 K, and the air is preheated to 423 K.
The heat capacities of the stack gas components may be assumed to have the following constant values in J/(mol.K): \(\ce{CO2}\) = 50; \(\ce{H2O(g)}\) = 38.5; \(\ce{O2}\) = 33.1; \(\ce{N2}\) = 31.3.
Standard heat of formation values in kJ/mol are as follows: \(\ce{CH4}\) = \(-74.85\); \(\ce{C2H6}\) = \(-84.67\); \(\ce{CO2}\) = \(-393.50\); \(\ce{H2O(g)}\) = \(-241.83\).- Assuming complete combustion of the fuel, calculate the adiabatic flame temperature.
- How would the flame temperature change if the percent excess air were increased?
- How would the flame temperature change if the percent methane in the fuel were increased?
-
Maximum Allowable Conversion of Exothermic Reaction Page2002-13-pcAmmonia is produced by the following reaction\[ \text{N}_2 + 3\text{H}_2 \rightleftharpoons 2\text{NH}_3 \]In a commercial process for ammonia production, the feed to an adiabatic reactor contains 1 kmol/s of nitrogen and stoichiometric amount of hydrogen at 700 K. What is the maximum allowable conversion (in percentage) in the reactor, if the adiabatic temperature rise across the reactor should not exceed 100 K. Assume the feed and product streams to be ideal gas mixtures. The heat of reaction at 700 K for the above reaction is calculated to be -94.2 kJ/mol. Mean molar heat capacities (\(C_P\)), in the range 700 - 800 K, are 0.03, 0.0289 and 0.0492 kJ/(mol.K) for nitrogen, hydrogen and ammonia respectively.
-
Heat Loss from the Combustion System Page2014-42-pc
Carbon monoxide (CO) is burnt in presence of 200% excess pure oxygen and the flame temperature achieved is 2298 K. The inlet streams are at 25oC. The standard heat of formation (at 25oC) of CO and CO2 are -110 kJ.mol-1 and -390 kJ.mol-1, respectively. The heat capacities (in J.mol-1.K-1) of the components are \[ C_{P_{\text{O}_2}}= 25 + 14\times10^{-3}T \qquad C_{P_{\text{CO}_2}} = 25 + 42\times10^{-3}T \] where, \(T\) is the temperature in K. The heat loss (in kJ) per mole of CO burnt is _______.
-
1997-13-pc
A feed at 1298 K, consisting of flue gas (\(\ce {CO2}\), \(\ce {O2}\) and \(\ce {N2}\)) and air, is passed through a bed of pure carbon. The reactions that occur both go to completion. \[ \begin {align*} \ce {CO2(g)} + \ce {C(s)} &\rightarrow 2\ce {CO(g)}, \quad \Delta H^\circ _R \text { at 298 K = 170 kJ/mol} \\
\ce {O2(g)} + 2\ce {C(s)} &\rightarrow 2\ce {CO(g)}, \quad \Delta H^\circ _R \text { at 298 K = \(-\)220.4 kJ/mol} \end {align*} \] The combustor is adiabatic and the product gases exit at 1298 K. Calculate the required moles of \(\ce {CO2}\) per mole of \(\ce {O2}\) in the feed stream, so that the net heat generated is zero and the bed temperature remains constant at 1298 K.
Data: Mean molar heat capacities, \(C_{Pm}\)Substance \(\ce{C}\) \(\ce{O2}\) \(\ce{CO}\) \(\ce{CO2}\) \(C_{Pm}\) (kJ/[mol.K]) 0.02 0.03 0.03 0.05 -
Calorific Value of Fuels Page
-
Heat Transferred from Reactor Page
-
Adiabatic Combustion of Methane Page
-
Heat Released from Combustion of CO Page
-
Rate of Heat Removal from Ammonia Oxidizer Page
-
Heat Removed from Ammonia Oxidizer - Multiple Reactions Page
Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Tow principal reactions occur: \[ \begin{align*} \ce{4NH3} + \ce{5O2} &\rightarrow \ce{4NO} + \ce{6H2O} \\ \ce{2NH3} + \ce{2/3 O2} &\rightarrow \ce{N2} + \ce{3H2O} \end{align*}\]
A flowchart of the reactor follows.
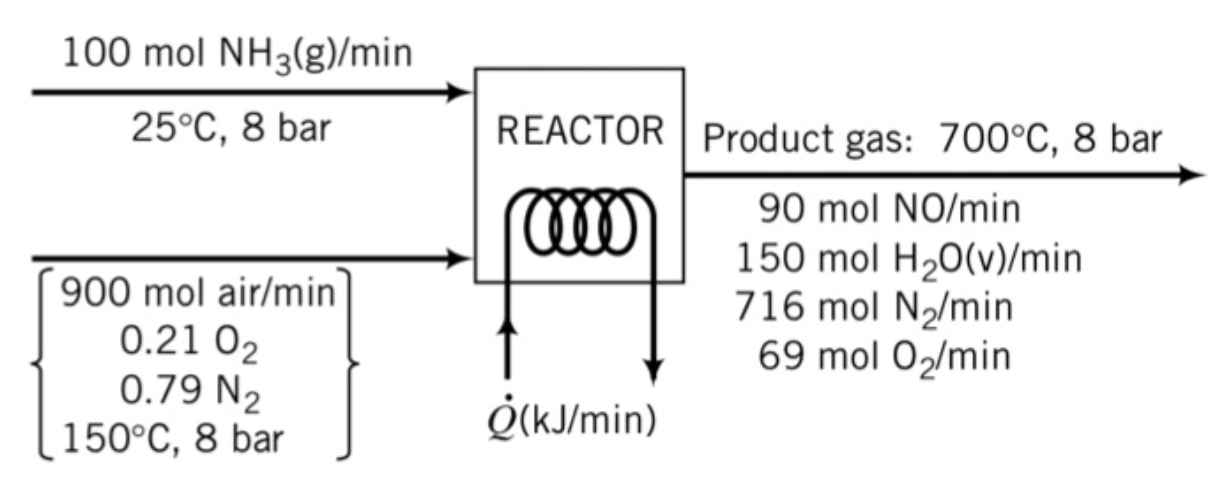
Mean specific heat and standard heat of formation of components are given in the table:
Component Cpm (25 to 150oC)
J/(mol.oC)Cpm (25 to 700oC)
J/(mol.oC)\(\Delta H_f^\circ\)
kJ/molNH3(g) 37.8 46.0 -46.19 NO(g) 30.2 32.0 90.37 H2O(g) 34.1 36.9 -241.83 O2(g) 30.1 32.4 - N2(g) 29.2 30.5 -
- Calculate the required heat transfer to or from the reactor in kW.
- Heat is to be ______ (removed from the reactor / added to the reactor)
-
Temperature of Ammonia Oxidizer Page
-
Energy Balance for Production of Acetylene - Another Explanation Page
-
Exit Temperature of Flue Gas Page
-
Excess Air and Adiabatic Flame Temperature Page
