Instant Notes
-
Azeotropes
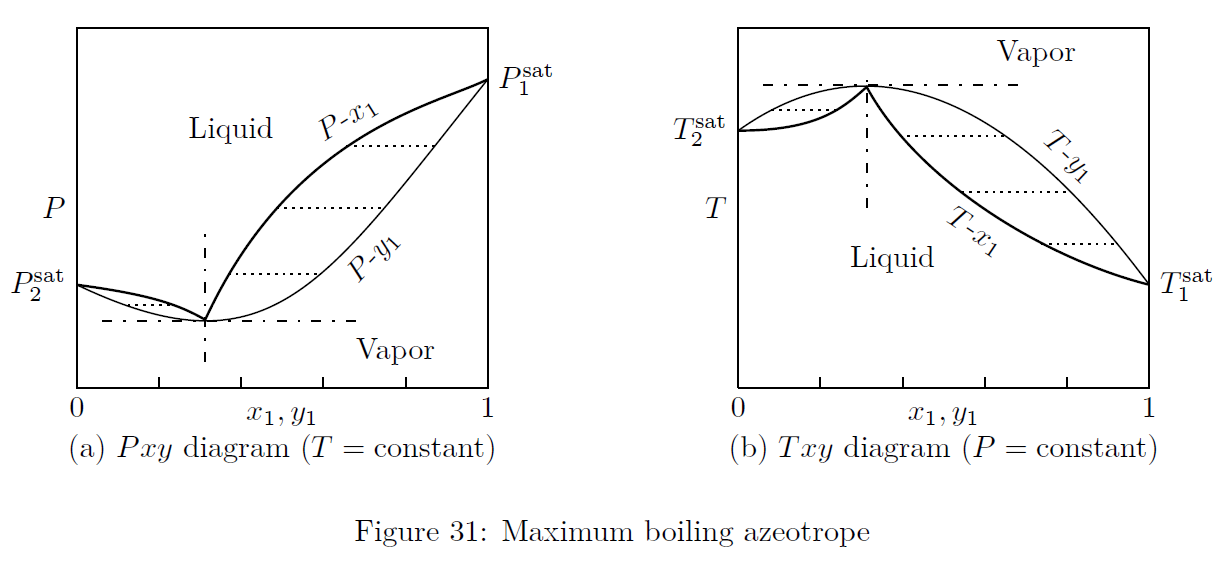
Azeotropes are constant boiling mixtures. When an azeotrope is boiled, the resulting vapor will have the same composition as the liquid from which it is produced. The thermodynamic diagrams of two different types of azeotropes are given in Figs.(30, 31).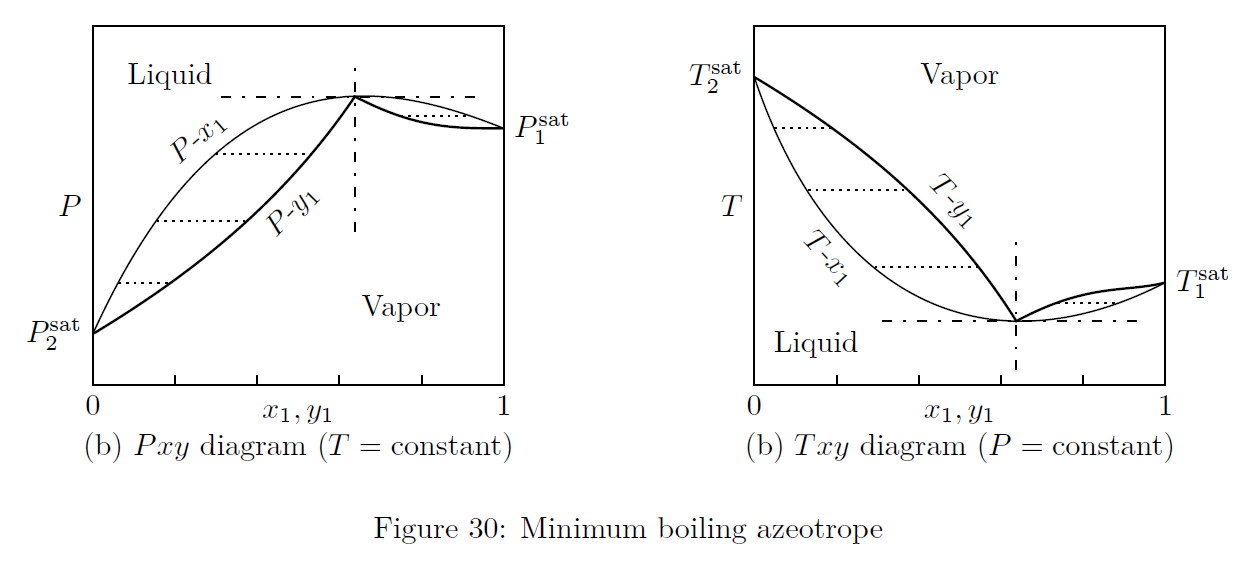
-
Minimum Boiling Azeotrope: High positive deviation from ideality leads to low boiling or minimum boiling azeotrope. At azeotropic composition, boiling point is lower than the boiling point of either components.
e.g.: Ethanol - water system. -
Maximum Boiling Azeotrope: High negative deviation from ideality leads to high boiling or maximum boiling azeotrope. At azeotropic composition, boiling point is higher than the boiling point of either components.
e.g.: HCl - water system.
-
