3. Chemical Process Industries
Sodium Carbonate
Solvay Process
Solvay process is based on the conversion of sodium chloride to sodium carbonate using ammonia and carbon dioxide.
\[\ce{NaCl} + \ce{NH3} + \ce{CO2} + \ce{H2O} \rightarrow \ce{NaHCO3} + \ce{NH4Cl}\] Ammoniated solution of salt is carbonated with \(\ce{CO2}\) from a coke-fired lime-kiln.
The precipitated sodium bicarbonate is converted to sodium carbonate by heating it, releasing water and carbon dioxide:
\[\ce{2NaHCO3} \rightarrow \ce{Na2CO3} + \ce{H2O} + \ce{CO2}\]
Ammonia is regenerated from the ammonium chloride byproduct by treating it with the milk of lime (calcium hydroxide) obtained from carbon dioxide generation:
\[\ce{2NH4Cl} + \ce{Ca(OH)2} \rightarrow \ce{2NH3} + \ce{CaCl2} + \ce{2H2O}\]
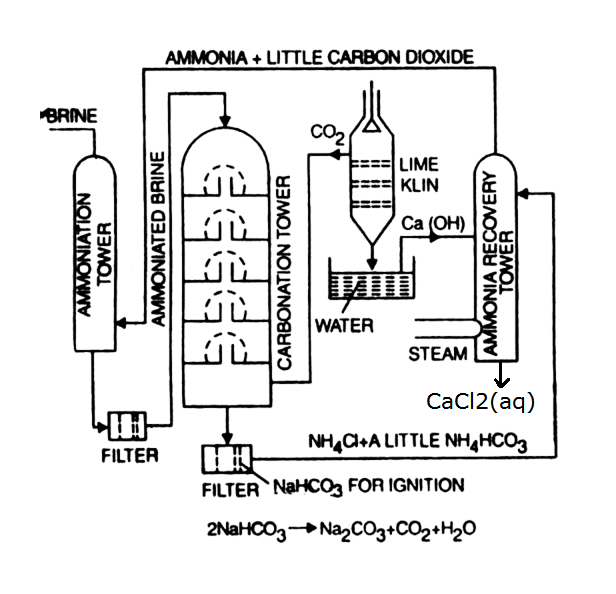
Recovery of Ammonia:
The lime-treated solution is fed to the top of the bubble-cap distillation unit. Steam is injected at the bottom, stripping out the ammonia down to a residual level of only 0.001 percent.
Calcination of \(\ce{NaHCO3}\):
The crude \(\ce{NaHCO3}\) is calcinated in dryers constructed with rotating seals and gas-tight feed and discharge mechanisms, to ensure the production of \(\ce{CO2}\) that is undiluted with air.
