3. Cooling Tower
Completion requirements
Cooling of Water in a Heat Exchanger
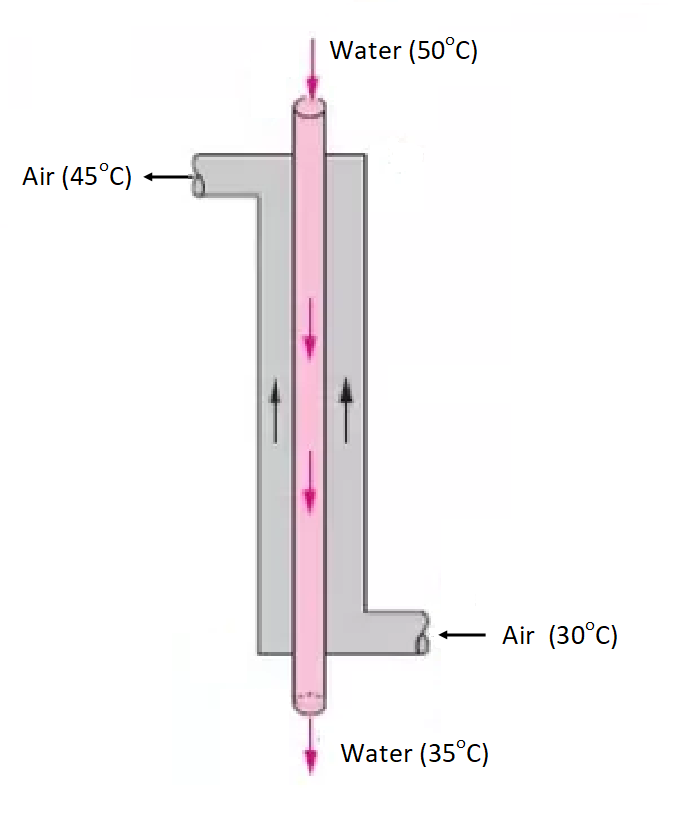
\[(mC_P\Delta T)_{\text{water}} = (mC_P\Delta T)_{\text{air}}\] Specific heat of water = 4.2 kJ/(kg.\(^\circ\)C)
Specific heat of air = 1 kJ/(kg.\(^\circ\)C)
For cooling of cooling-water with air in a heat exchanger, i.e., by indirect contacting of water with air, from energy balance, we can prove that 4.2 kg of air is needed for for 1 kg of water.
Density of water = 1000 kg/m\(^3\)
Density of air \(\approx\) 1 kg/m\(^3\)
Air being lighter, we need to circulate a large volume of air — 4200 ltr of air per ltr of water.
