Reaction Engineering - Video Lectures
Topic outline
-
-
Mole Balance in Multiple Reactions Page1993-13-b-creFor multiple reactions \[ \begin {align*} 2A &\rightarrow R \\
2R &\rightarrow S \end {align*} \] the number of moles of \(S\) present when the number of moles of \(A\) and \(R\) are 0.3 and 0.5 respectively (Initially 2 moles of \(A\) are only present) are ________ (0.125 / 0.175 / 0.350 / 0.535)
Enrol me in this course -
Selectivity of Parallel Reactions Page2000-1-20-creFor the liquid phase parallel reactions \[ \begin {align*} A &\rightarrow R& r_R &= k_1C_A^2;& E_1 &= 80 \text { kJ/mol} \\
A &\rightarrow S& r_S &= k_2C_A;& E_2 &= 120 \text { kJ/mol} \end {align*} \] the desired product is \(R\). A higher selectivity of \(R\) will be achieved if the reaction is conducted at- low temperature in a CSTR
- high temperature in a CSTR
- low temperature in a PFR
- high temperature in a PFR
-
Maximizing the Yield with 3 Parallel Reactions Page2011-22-creReactant \(R\) forms three products \(X\), \(Y\), and \(Z\) irreversibly, as shown below.
The reaction rates are given by \(r_X=k_XC_R\), \(r_Y=k_YC_R^{1.5}\) and \(r_Z=k_ZC_R\). The activation energies for formation of \(X\), \(Y\), and \(Z\) are 40, 40 and 5 kJ/mol respectively. The pre-exponential factors for all reactions are nearly same. The desired conditions for MAXIMIZING the yield of \(X\) are
- high temperature, high concentration of \(R\)
- high temperature, low concentration of \(R\)
- low temperature, high concentration of \(R\)
- low temperature, low concentration of \(R\)
-
Parallel Reaction in MFR PageLevenspiel3E-7-8Liquid reactant \(A\) decomposes as follows:
A feed of aqueous \(A\) (\(C_{A0}=40\) mol/m3) enters a mixed flow reactor, decomposes, and a mixture of \(A, R\) and \(S\) leaves. Find \(C_R\) and \(C_S\) and \(\tau\) for \(X_A=0.9\).
Enrol me in this course -
Parallel Reactions in MFRs in Series - First Order PageLevenspiel3E-7-6Substance \(A\) in a liquid reacts to produce \(R\) and \(S\) as follows:
A feed (\(C_{A0} = 1 \text{ mol/liter}, C_{R0} = 0 \text{ mol/liter}, C_{S0} = 0 \text{ mol/liter}\)) enters two mixed flow reactors in series, with \(\tau_1=2.5 \text{ min}\) and \(\tau_2=5 \text{ min}\). Knowing the composition in the first reactor (\(C_{A1}=0.4 \text{ mol/liter}, C_{R1}=0.4 \text{ mol/liter}, C_{S1}=0.2 \text{ mol/liter}\)), find the composition leaving the second reactor.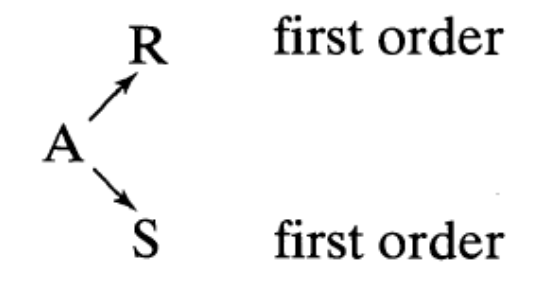
Enrol me in this course -
Parallel Reactions in MFRs in Series PageLevenspiel3E-7-7Substance \(A\) in the liquid phase reacts to produce \(R\) and \(S\) as by the following reactions:
The feed (\(C_{A0} = 1 \text{ mol/liter}, C_{R0} = 0 \text{ mol/liter}, C_{S0} = 0.3 \text{ mol/liter}\)) enters two mixed flow reactors in series, with \(\tau_1=2.5 \text{ min}\) and \(\tau_2=10 \text{ min}\). Knowing the composition in the first reactor (\(C_{A1}=0.4 \text{ mol/liter}, C_{R1}=0.2 \text{ mol/liter}, C_{S1}=0.7 \text{ mol/liter}\)), find the composition leaving the second reactor.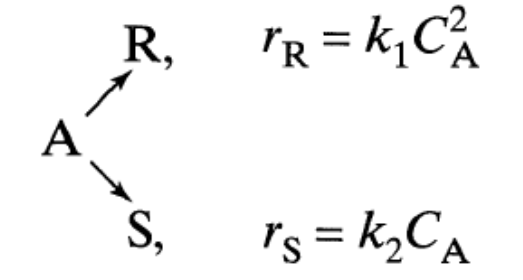
Enrol me in this course -
Levenspiel3E-7-9Liquid reactant \(A\) decomposes as follows:
A feed of aqueous \(A\) (\(C_{A0}=40\) mol/m3) enters a plug flow reactor, decomposes, and a mixture of \(A, R\) and \(S\) leaves. Find \(C_R\) and \(C_S\) and \(\tau\) for \(X_A=0.9\).
Enrol me in this course -
Parallel Reactions in PFR - Rate Constants for the given Product Distribution Page
-
Temperature of PFR for the given Product Distribution Page
-
Parallel Reactions in Batch Reactor Page
-
Conditions Maximizing a Parallel Reaction in MFR Page
-
Parallel Bimolecular Reactions in MFR Page
-
Parallel Reactions in CSTR - First and Second Order Reactions Page
-
Maximizing the Desired Product - 3 Parallel Reactions in MFR Page
-
Maximizing the Yield with Series Reactions Page
-
Series Reactions in PFR and MFR Page
-
Series Reactions in PFR and MFR - Example-2 Page
-
Series Reactions in Batch Reactor - First Order followed by Zeroth Order Page
-
